Rights Issue: Gabather
- Aktiekurs
- 0.03
- Bransch
- Pharmaceuticals
- Lista
- Nasdaq First North
- Emissionsvolym
- 21,4 MSEK
- Teckningskurs
- 0,03 SEK
- Teckningsperiod
- 4 - 18 mars
- Första handelsdag
- N/A
- Garanti- och teckningsåtagande
- 0 %
AKTIE 0,03 SEK
INVESTERING N/A
VALUATION 0,723 MSEK
Gabather AB (“Gabather” or “the Company”) is advancing GT-002, a first-in-class GABAA-modulator, through Phase II clinical trials, targeting cognitive dysfunction. With a fully funded schizophrenia Phase II trial and a planned expansion into Phase II addressing dementia, the Company addresses a multi-billion-dollar CNS market with high unmet medical need. Gabather’s differentiated mechanism of action, strong patent portfolio, broad indication potential and expanding research efforts positions the Company to drive long-term value creation. The Company is in discussions with potential partners, further strengthening Gabather’s commercial prospects. Analyst Group considers the ongoing rights issue a compelling opportunity with an attractive Risk/Reward profile at a Pre-Money valuation of SEK 713.2k.
Gabather is a clinical-stage biotech company developing innovative treatments for neuropsychiatric disorders. The Company’s research focuses on GABAA receptors, a key component of the brain’s signalling system, where imbalances are linked to conditions such as schizophrenia, dementia, depression, psychosis, and Alzheimer’s disease.
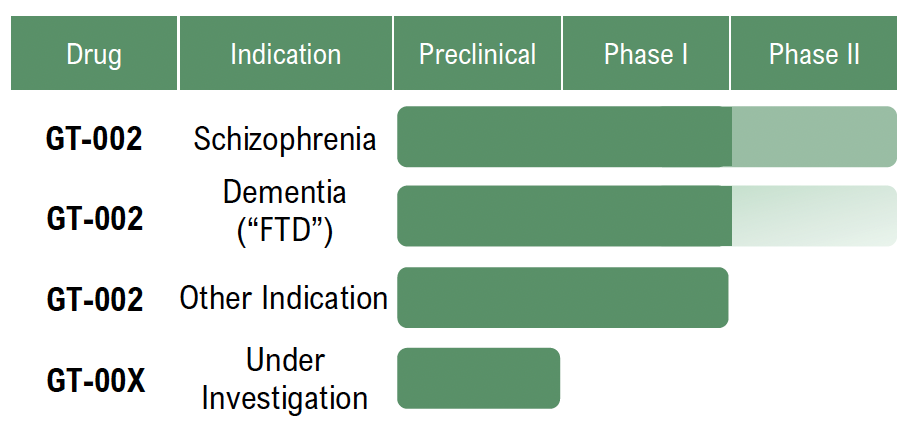
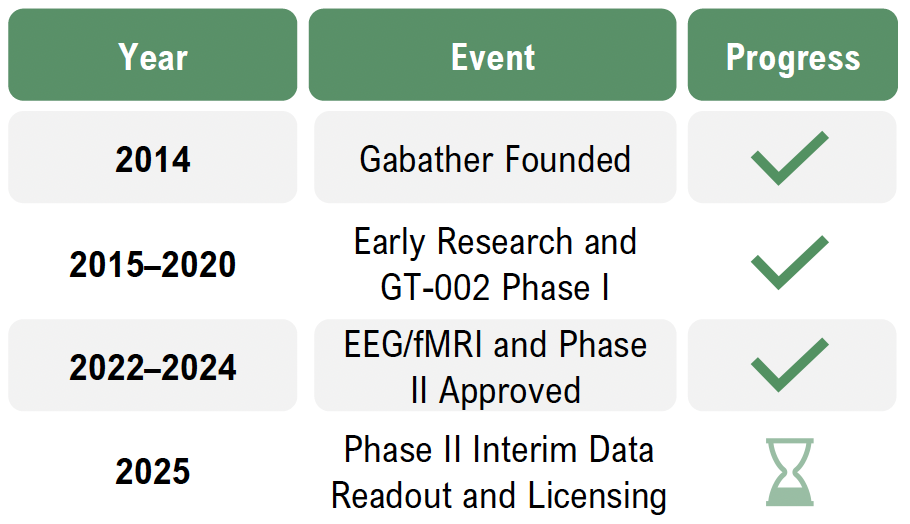
Unlike traditional treatments that often have slow onset, severe side effects, or limited efficiency, Gabather’s proprietary drug platform aims to enable the development of small-molecule compounds that selectively modulate GABAA receptors. This approach aims to provide faster, more targeted, and safer treatments. The Company’s lead candidate, GT-002, is in Phase II clinical trials addressing schizophrenia, which is fully financed via Innovationsfonden in Denmark, and has shown promising results in preclinical and previous clinical studies.
Furthermore, Gabather is advancing research into further CNS disorders, including the planned development of GT-002 for dementia. With a strong patent portfolio protecting the technology in key markets, Gabather is focused on advancing the Company’s clinical programs and forming strategic partnerships for out-licensing oppor-tunities.
GT-002 is Gabather’s lead drug candidate, designed to selectively modulate GABAA receptors for the treatment of neuropsychiatric disorders. Unlike traditional treatments, GT-002 aims to provide faster symptom relief with fewer side effects by targeting specific receptor subtypes linked to cognition and emotional regulation.
The drug has successfully completed a comprehensive Phase I clinical program, demonstrating excellent safety, tolerability, and pharmacokinetics. In 2019, a randomized, double-blind, placebo-controlled Phase I-a study in 32 healthy volunteers confirmed GT-002’s safety and strong absorption. A Phase I-b study in 2020 further validated the treatment’s tolerability in repeated dosing.
Currently, GT-002 is in Phase II trials, evaluating its cognitive and therapeutic effects in schizophrenia. Early results suggest that GT-002 can enhance brain activity and cognitive function, positioning the treatment as a potential breakthrough in neuropsychiatric treatment.
Strong Clinical Potential
Gabather’s lead candidate, GT-002, is in Phase II clinical trials addressing schizophrenia which is fully financed. Gabather’s dual-market strategy, targeting both schizophrenia and FTD, diversifies the clinical pipeline, reducing developmental risk while enhancing commercial upside. If successful, GT-002 could pioneer a new treatment paradigm in cognitive restoration, with applications across multiple disorders.
Large and Underserved Market
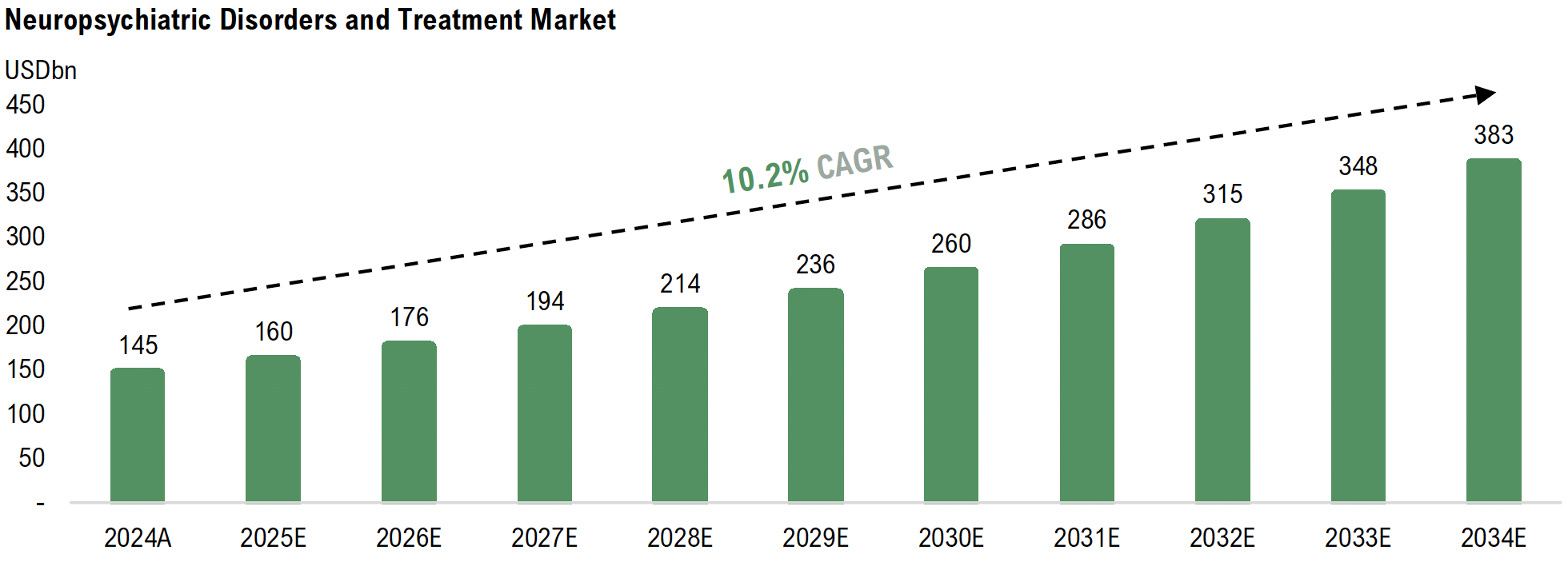
With GT-002, Gabather targets the market for neuro-psychiatric disorders and treatments, estimated to grow at a CAGR of 10.2% from 2025 to 2034, reaching a market value of USD 383bn with an estimated TAM of 320 million patients worldwide. The high unmet medical needs positions Gabather to capture significant value as demand for safer and more effective therapies grow.
Partnership and Licensing Potential
With GT-002 in Phase II clinical trials and a differentiated approach to neuropsychiatric treatment, Gabather is well-positioned to attract partnerships with major pharmaceutical companies. The CNS market has seen significant licensing deals for innovative treatments – Gabather’s strong preclinical and clinical data makes the Company an attractive candidate for partnerships and/or out-licensing opportunities.
Gabather is advancing the Company’s innovative GABAA-modulating technology through both clinical development and discussions with potential partners. With GT-002 in Phase II trials, the Company is generating critical data to support the potential of a next-generation treatment for neuropsychiatric disorders. The ongoing Phase II study is a double-blind, randomized and placebo-controlled trial evaluating GT-002’s effects on cognition and neuropsychiatric symptoms in schizophrenia patients, aiming to validate GT-002’s cognitive and therapeutic benefits, further strengthening Gabather’s data and position in a market with high unmet medical needs. Beyond clinical advancements, Gabather is actively working to expand the strategic network through partnerships and licensing opportunities. The Company’s fully funded Phase II study with CNSR in Denmark highlights the growing interest in the GT-002 mechanism and strengthens the discussions with potential pharmaceutical partners. By combining a strong patent portfolio, differentiated drug profile, and solid clinical momentum, Gabather is well-positioned to unlock new opportunities for collaborations with potential partners and future licensing agreements.
To advance the Company’s clinical and business development efforts, Gabather is conducting a rights issue expected to raise up to SEK 18.25m in net proceeds. The proceeds will enable the Company to continue the Phase II trial of GT-002 for dementia (“FTD”), explore new indications, and further develop the drug pipeline. Furthermore, the proceeds are expected to fund several key milestones throughout 2025:
- Q1 2025: Launch of the TOTEMS Phase II study for schizophrenia and expanded EEG/fMRI data analysis.
- Q2 2025: Preclinical data readout for new candidates.
- Q3 2025: Interim results from the TOTEMS study.
- Q4 2025: Potential licensing agreement for GT-002
Additionally, the financing will support ongoing business development efforts, reinforcing the Company’s position in discussions with potential pharmaceutical partners.
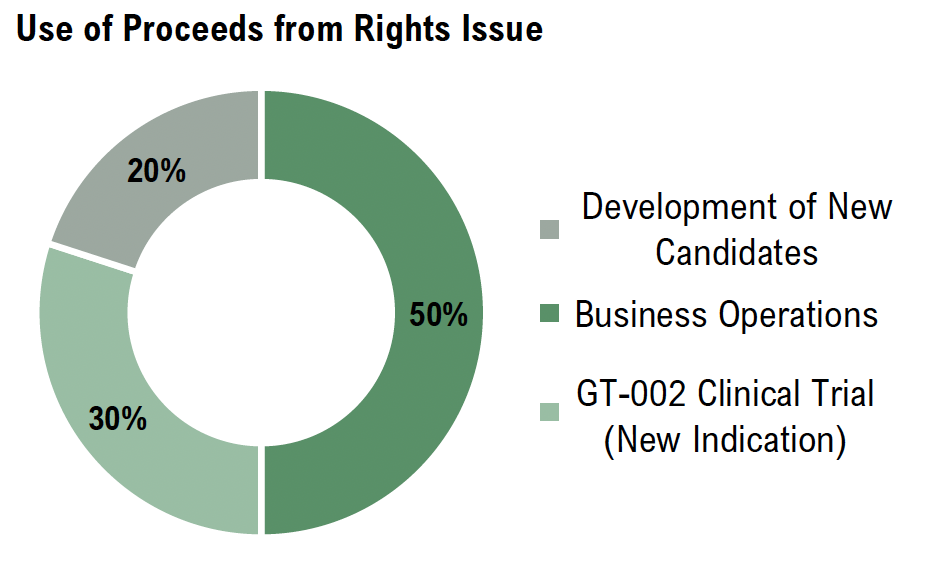
The proceeds from the rights issue will enable Gabather to advance the clinical and preclinical programs for new indications while strengthening the Company’s business development efforts. A significant portion will support the continued development of GT-002, including preparations for Phase II in FTD. Additionally, resources will be allocated to developing the next-generation drug candidate, ensuring a robust pipeline for future growth. These efforts position Gabather to drive clinical progress, strategic partnerships, and long-term value creation.
The TOTEMS study (Targeting Oscillations in the Excitation-Inhibition Balance in Schizophrenia) is a Phase II clinical trial designed to evaluate the effects of GT-002 on cognitive function and neurophysiological activity in patients diagnosed with schizophrenia. The study is being conducted in collaboration with the Centre for Neuro-psychiatric Schizophrenia Research (“CNSR”) at Psychiatric Centre Glostrup in Denmark and is fully funded by Innovationsfonden in Denmark.
The TOTEMS study is a randomized, double-blind, placebo-controlled trial that aims to assess how GT-002 affects brain activity, cognition, and symptom severity in schizophrenia patients.
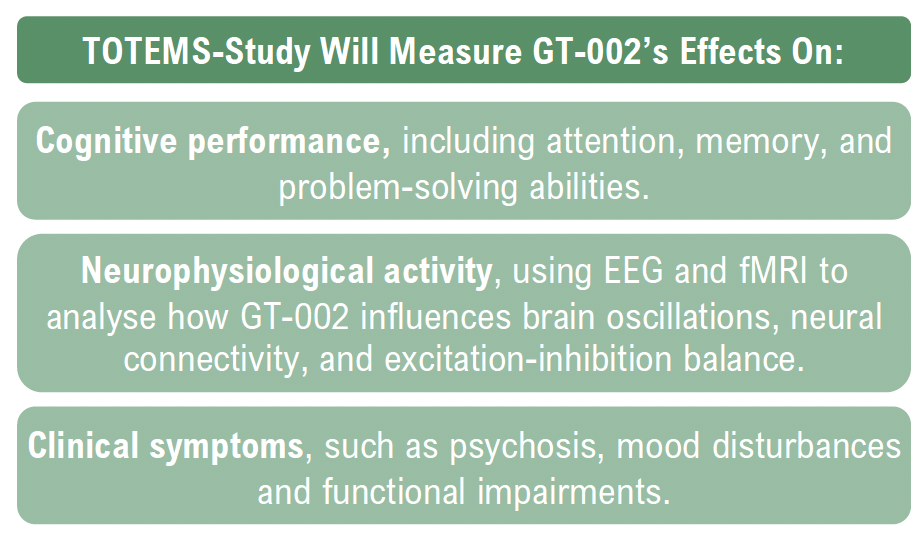
Cognitive deficits are a core symptom of schizophrenia that significantly impact daily functioning and quality of life, such as difficulties in maintaining employment, managing personal relationships, and performing everyday tasks. Existing antipsychotic treatments primarily address hallucinations and delusions but offer limited benefits for cognitive dysfunction. By targeting GABAA receptor modulation, GT-002 presents a mechanism of action that aims to improve cognitive function without the sedative effects commonly associated with current treatments.
If successful, the TOTEMS study would validate GT-002’s potential as a first-in-class treatment for cognitive impairment in schizophrenia, opening the door for future regulatory approvals and commercial partnerships.
This study is a key value-driving milestone for Gabather, as positive results significantly strengthens the Company’s position in partnering discussions and future licensing agreements.
In addition to schizophrenia, Gabather is preparing to evaluate GT-002 for the treatment of frontotemporal dementia (“FTD”), a neurodegenerative disorder characterized by progressive decline in behavior, personality, and language abilities. FTD currently has no approved disease-modifying treatments, making it an area of high unmet medical need.
The planned study aims to assess GT-002’s potential cognitive and neuropsychiatric benefits in FTD patients by evaluating:
- Cognitive function, particularly memory, executive function, and attention.
- Behavioural and psychiatric symptoms, including apathy, agitation, and mood disturbances.
- Neurophysiological biomarkers, using EEG and imaging techniques to monitor changes in brain activity.
Although the exact study design has not yet been disclosed, it is expected to be a randomized, placebo-controlled trial similar to the TOTEMS study in schizophrenia.
FTD is strongly associated with dysfunction in the GABA-ergic system, which regulates excitation and inhibition in the brain. By selectively modulating GABAA receptors, GT-002 has the potential to restore neural balance, improving both cognitive and behavioral symptoms in FTD patients.
Preclinical studies have already demonstrated pro-cognitive effects of GT-002, supporting the treatment’s potential use in neurodegenerative conditions. If successful, this study would position GT-002 as a novel treatment option in a market with limited therapeutic alternatives.
The study is currently in the planning phase, with initial funding set to stem from Gabather’s ongoing rights issue. Given full subscription, approximately 30% of the proceeds, corresponding to SEK 5.5m, will be allocated towards planning and initiating the trial. However, additional funding may be required to fully complete the study, depending on its scale and design.
This study represents an important expansion of GT-002’s therapeutic potential beyond psychiatric disorders and into the neurodegenerative space, increasing Gabather’s TAM and licensing potential.

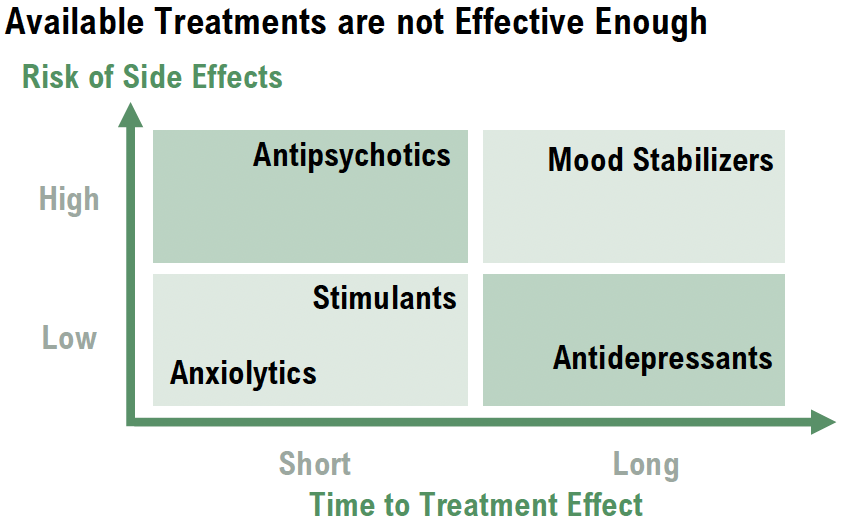
The market for schizophrenia treatments is undergoing a fundamental transformation. While traditional dopaminergic and serotonergic-based antipsychotics remain the standard of care, their inability to address cognitive impairment and negative symptoms has created a significant unmet medical need. Cognitive dysfunction, which affects memory, attention, and executive function, is one of the strongest predictors of long-term functional recovery, yet current therapies fail to provide meaningful improvement in this area. As a result, patients continue to experience reduced independence, lower employment rates, and an overall diminished quality of life, even when their positive symptoms are well-controlled.
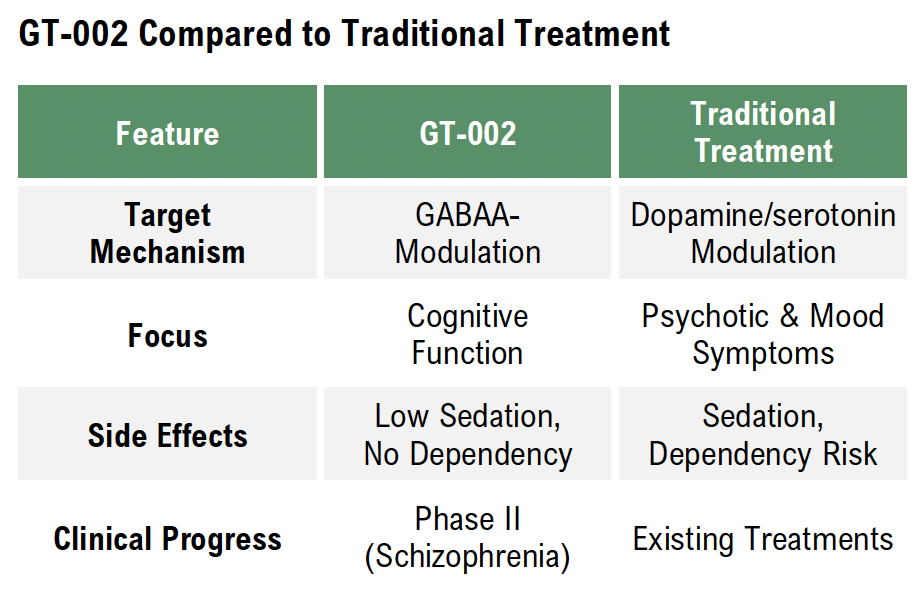
A growing body of research underscores the role of GABAergic modulation in cognitive enhancement, positioning GABAA receptor targeting as a viable alternative to dopamine- and serotonin-based therapies. The GABAA receptor plays a crucial role in inhibitory neuro-transmission, and dysregulation in this system has been linked to cognitive deficits in schizophrenia and other neuropsychiatric conditions. GT-002 directly modulates the GABAA receptor, offering a mechanism-driven approach to restoring cognitive function while avoiding the sedation, metabolic disturbances, and dependency risks associated with existing medications. Unlike traditional antipsychotics, which focus on managing hallucinations and delusions, GT-002 is designed to enhance cognitive processing, improve functional independence, and optimize patient outcomes, aligning with the industry’s increasing focus on real-world recovery metrics rather than just symptom suppression.
Gabather is positioned to capitalize on a potential paradigm shift in schizophrenia treatment with GT-002, a first-in-class GABAA receptor modulator that directly targets cognitive impairment. With the fully funded Phase II clinical trial for schizophrenia, and the Phase II trial planned in dementia, the Company is set to generate robust clinical data that will establish GT-002’s potential as a transformative therapy.
The TOTEMS study, which is fully funded by Innovationsfonden Denmark, is designed to evaluate GT-002’s impact on EEG biomarkers in schizophrenia patients, providing objective neurophysiological evidence of the drug’s mechanism of action. In parallel, another Phase II trial will assess GT-002’s effects on cognitive and functional outcomes in FTD patients, intended to demonstrate the drug’s potential to improve real-world quality of life for individuals affected by the disease. The study addressing FTD is intended to be partially financed through the Company’s ongoing rights issue.
If GT-002 demonstrates clinical effectiveness, it could become the first approved medication specifically designed to address cognitive dysfunction in schizophrenia, an area with no existing treatment options. This would position Gabather at the forefront of innovation in a field with significant unmet medical need. Furthermore, regulatory agencies are increasingly prioritizing cognitive outcomes, and pharmaceutical companies are actively seeking differentiated treatments in CNS disorders, creating a favourable environment for novel therapies like Gabather’s GT-002. With a fully funded Phase II schizophrenia study underway, the next 12 to 24 months will be critical in determining GT-002’s clinical potential and the treatments pathway toward regulatory approval. Positive data could open the door for strategic partnerships and accelerate commercialization efforts, positioning Gabather as a key player in the evolution of schizophrenia treatment.
The global schizophrenia drug market is set for substantial growth, driven by an increased emphasis on mental health treatment, regulatory incentives for innovation, and a shift in clinical priorities toward cognitive outcomes. While the broader neuropsychiatric drug market is projected to expand from USD 145 billion in 2024 to USD 383 billion by 2034¹, cognition-enhancing therapies are expected to grow at a CAGR of 7.1% from 2021 to 2029², as research and clinical focus increasingly recognize cognitive improvement as a core treatment objective. The need for novel, non-dopaminergic approaches that go beyond symptom control is a clear priority for both healthcare providers and regulatory agencies, which Gabather is set to capitalize on.
The global neuropsychiatric drug market is expanding rapidly, projected to grow at a CAGR of 10.2% until 2034 and reach USD 383 billion the same year¹, fuelled by aging populations, increased healthcare investment, and a renewed emphasis on neurodegenerative disease innovation. Within this high-growth market, FTD remains one of the most overlooked and underserved indications, creating an opportunity for novel, first-in-class therapies that can fill the current treatment void, such as Gabather’s GT-002.
Beyond schizophrenia, Gabather is strategically targeting frontotemporal dementia (“FTD”), a rapidly progressing neurodegenerative disease with no approved treatments. Unlike Alzheimer’s disease, which primarily affects memory, FTD leads to severe disruptions in personality, emotional regulation, and executive function, placing an immense burden on patients, caregivers, and healthcare systems. As the second most common cause of early-onset dementia, FTD presents an urgent clinical need that remains largely unaddressed by the pharmaceutical industry, where historical research efforts have been concentrated on Alzheimer’s disease and amyloid-targeting approaches. With the innovative GABAA-modulating mechanism, Gabather aims to offer a novel treatment approach that could help bridge this critical gap.
Gabather is pursuing Orphan Drug Designation (“ODD”) for GT-002 in FTD, which would provide market exclusivity, financial incentives, and expedited regulatory review. If granted, ODD status would strengthen GT-002’s commercial positioning, making it a strategic asset for partnerships, licensing deals, and non-dilutive funding opportunities. Additionally, the FDA and EMA’s prioritization of neurodegenerative research has opened the door for Fast Track and Breakthrough Therapy designations, potentially accelerating GT-002’s development timeline and enhancing its market entry prospects.
Scientific advances in GABAergic modulation indicate that restoring inhibitory neurotransmission in FTD could play a key neuroprotective role, preventing the circuit-level disruptions that drive disease progression. By applying the clinical insights gained from schizophrenia trials, Gabather aims to expand GT-002’s applicability into FTD and broader neurodegenerative indications, positioning it as a platform technology spanning multiple high-value CNS markets.
Gabather’s dual-market strategy, targeting both schizophrenia and FTD, diversifies the clinical pipeline, reducing developmental risk while enhancing commercial upside. If successful, GT-002 could pioneer a new treatment paradigm in cognitive restoration, with applications across psychiatric and neurodegenerative disorders.
With near-term Phase II data in schizophrenia, upcoming interim results, and ongoing regulatory initiatives in FTD, the next 12 to 24 months will be pivotal in determining GT-002’s potential to redefine treatment standards across multiple CNS segments. Key value drivers include patient recruitment milestones, interim efficacy readouts, and potential partnership discussions. Gabather’s approach aligns with scientific advancements, regulatory priorities, and industry demand for innovation, positioning the Company at the forefront of one of the most pressing challenges in modern neuro-science.
Unlike traditional therapies that primarily focus on managing symptoms such as psychosis or mood disturbances, GT-002 is designed to directly target cognitive dysfunction, one of the most debilitating and overlooked aspects of neuropsychiatric disorders. By selectively modulating GABAA receptors, GT-002 offers a more precise and safer mechanism of action, aiming to restore excitation-inhibition balance in the brain, a fundamental process disrupted in conditions like schizophrenia and dementia.
Early clinical and preclinical data indicate promising effects on brain activity, cognition, and emotional regulation, reinforcing GT-002’s potential to become a differentiated and clinically superior alternative to current treatments. Its high selectivity minimizes common side effects such as sedation and dependency, addressing a critical gap in the market. Furthermore, the ongoing fully funded Phase II trial in schizophrenia provides an important de-risking milestone, as it generates valuable clinical data that will support future regulatory advancements and commercial discussions.
Additionally, Gabather is advancing a pipeline of CNS-targeted candidates, including the exploration of GT-002 in other indications such as FTD, further enhancing the longterm potential and TAM.
The neuropsychiatric treatment market represents one of the largest and most underserved segments in the pharmaceutical industry, with over 970 million people worldwide suffering from mental health disorders. Despite the widespread prevalence of conditions such as schizophrenia, depression, and FTD, existing treatments often provide limited efficacy, slow action onset, and severe side effects. This leaves a substantial commercial gap and a growing demand for safer, more effective therapies, creating an opportunity for new entrants to disrupt the market.
Gabather is positioned to capitalize on this demand. With GT-002 and additional drug candidates in development, the Company is targeting a CNS market expected to reach USD 383 billion by 2034, driven by an aging populations, increased mental health awareness, and a shift towards novel mechanisms of action.
Unlike existing therapies that primarily suppress symptoms, GT-002’s selective GABAA receptor modulation addresses both psychiatric and cognitive symptoms, enabling a broad market potential across multiple indications. The combination of a differentiated mechanism of action, a rapidly growing market, high unmet medical need and a first-mover advantage in a promising treatment approach, positions the Company to drive significant value creation as Gabather’s clinical programs progress.
A key driver of value in the biotech sector is the ability to attract strategic partnerships and licensing agreements with major pharmaceutical companies. The CNS market has seen a surge in high-value deals, as large pharma seeks to expand their pipelines with innovative treatments addressing high unmet medical needs. Gabather, with GT-002 in Phase II clinical trials and a strong patent portfolio, is in a prime position to capitalize on this trend.
Successful Phase II data is often a critical inflection point where biotech companies secure licensing or co-development deals, unlocking milestone payments, royalties, and non-dilutive capital. With GT-002 advancing in clinical development, the likelihood of a licensing agreement within the next year increases, positioning Gabather for significant commercial validation.
Additionally, Gabather’s fully funded schizophrenia trial and planned expansion into new indications enhance the attractiveness of GT-002 as a partnership opportunity. As clinical progress continues, the Company’s unique approach to GABAA modulation and ability to target multiple neuropsychiatric disorders strengthens Gabather’s position in discussions with potential partners. With an active focus on business development, Gabather is well-positioned to secure value-generating agreements.
In conclusion, Gabather presents an interesting biotech investment at a pivotal stage. With GT-002 in Phase II trials, the Company targets a large unmet medical need. The fully funded Phase II schizophrenia study enhance the likelihood of a licensing agreement within the next year, positioning Gabather for significant value creation. All together, Analyst Group views Gabather, at a Pre-Money valuation of SEK 713.2k in conjunction with the rights issue, to have an attractive Risk/Reward profile.
Dessa analyser, dokument eller annan information härrörande AG Equity Research AB (vidare AG) är framställt i informationssyfte, för allmän spridning, och är inte avsett att vara rådgivande. Informationen i analyserna är baserade på källor och uppgifter samt utlåtanden från personer som AG bedömer vara tillförlitliga. AG kan dock aldrig garantera riktigheten i informationen. Alla estimat i analyserna är subjektiva bedömningar, vilka alltid innehåller viss osäkerhet och bör användas varsamt. AG kan därmed aldrig garantera att prognoser och/eller estimat uppfylls. Detta innebär att investeringsbeslut baserat på information från AG eller personer med koppling till AG, alltid fattas självständigt av investeraren. Dessa analyser, dokument och information härrörande AG är avsett att vara ett av flera redskap vid investeringsbeslut. Investerare uppmanas att komplettera med ytterligare material och information samt konsultera en finansiell rådgivare inför alla investeringsbeslut. AG frånsäger sig allt ansvar för eventuell förlust eller skada av vad slag det må vara som grundar sig på användandet av material härrörande AG. Läsare kan anta att Analyst Group har mottagit ersättning för att framställa detta material.
Nordnet eller Avanza har inte varit part i framtagandet av erbjudandet, utan mottar och administrerar endast ansökningar om teckning.




